
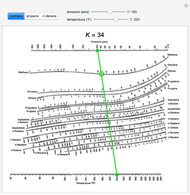
Such VLE information is useful in designing columns for distillation, especially fractional distillation, which is a particular specialty of chemical engineers. In certain cases such VLE data can be determined or approximated with the help of certain theories such as Raoult's Law, Dalton's Law, and/or Henry's Law. Such VLE concentration data is often known or can be determined experimentally for vapor-liquid mixtures with various components. The equilibrium concentration of each component in the liquid phase is often different from its concentration (or vapor pressure) in the vapor phase, but there is a correlation. This fact is true in reverse also if a vapor with components at certain concentrations or partial pressures is in vapor-liquid equilibrium with its liquid, then the component concentrations in the liquid will be set dependent on the vapor concentrations, again also depending on the temperature. At vapor-liquid equilibrium, a liquid with individual components (compounds) in certain concentrations will have an equilibrium vapor in which the concentrations or partial pressures of the vapor components will have certain set values depending on all of the liquid component concentrations and the temperature. The equilibrium vapor pressure of a liquid is usually very dependent on temperature. The concentration of a vapor in contact with its liquid, especially at equilibrium, is often given in terms of vapor pressure, which could be a partial pressure (part of the total gas pressure) if any other gas(es) are present with the vapor. 3.1 K values and relative volatility values.2 Thermodynamic description of vapor-liquid equilibrium.Although in theory equilibrium takes forever to reach, such an equilibrium is practically reached in a relatively closed location if a liquid and its vapor are allowed to stand in contact with each other long enough with no interference or only gradual intereference from the outside.
Vapor-liquid equilibrium, abbreviated as VLE by some, is a condition where a liquid and its vapor (gas phase) are in equilibrium with each other, a condition or state where the rate of evaporation (liquid changing to vapor) equals the rate of condensation (vapor changing to liquid) on a molecular level such that there is no net (overall) vapor-liquid interconversion.


 0 kommentar(er)
0 kommentar(er)
